Answers
Answer:
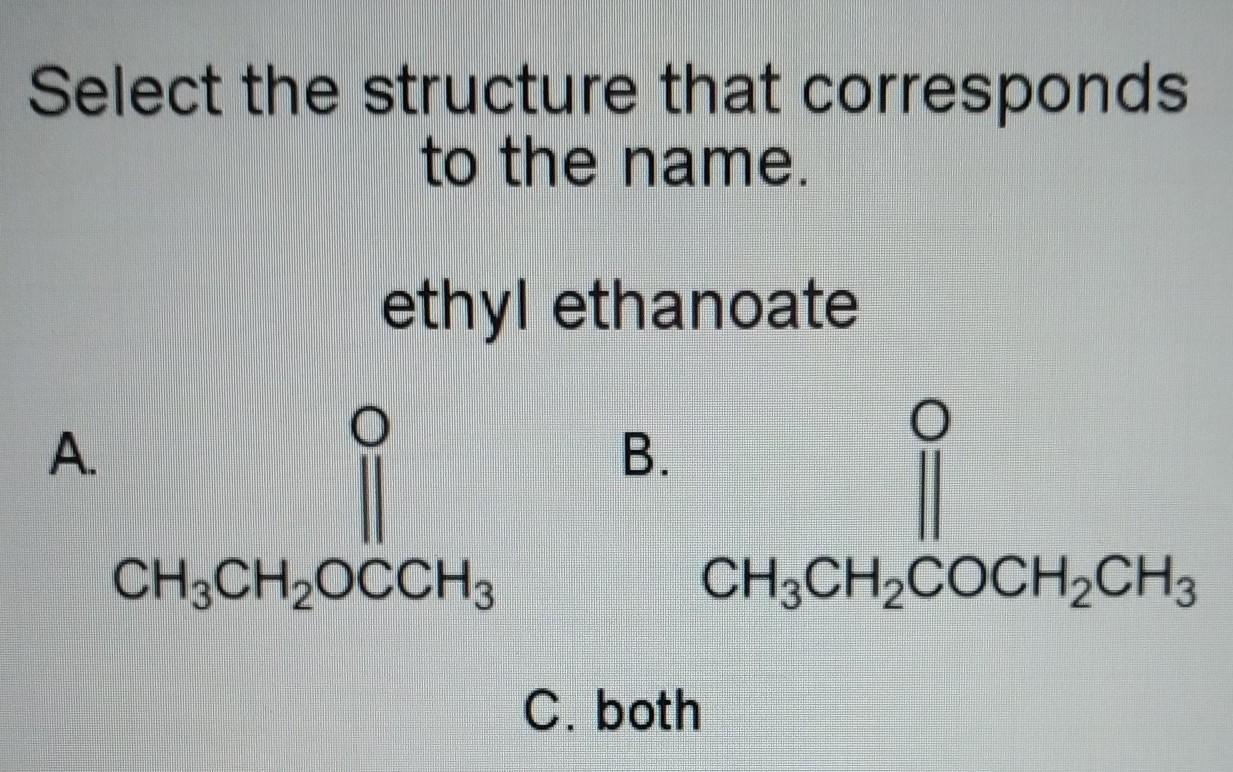
i think that it is C. both
Explanation:
Related Questions
What must occur for the enthalpy of solution to be negative?
The process of separating the solvent particles from one another must release energy.
The process of separating the solute particles from one another must release energy.
More energy is absorbed as the solute and solvent mix than is released when the solute particles and the solvent particles separate.
More energy is released as the solute and solvent mix than is absorbed when the solute particles and the solvent particles separate.
Answers
Answer:
d on edge my guys.
Explanation:
Mg + _________HCI →
_____ H₂ + _____ MgCh
Answers
Mg + 2HCl → H2 + MgCl2 because The reaction between magnesium and hydrochloric acid produces hydrogen gas and magnesium chloride.
What is molecule?Molecules are the smallest unit of a chemical compound that can exist independently and remain unchanged in chemical composition. A molecule is composed of two or more atoms that are chemically bonded together. A molecule can be made up of different elements, such as carbon, hydrogen, oxygen, and nitrogen, and can be composed of a variety of combinations of these elements. Molecules are essential components of all living things, as they are important for basic functions such as energy transfer, metabolism, and cell structure.
To learn more about molecule visit:
brainly.com/question/475709
#SPJ1
The zeroth law of thermodynamics describes the state where we can say what about the movement of heat between two substances?
a. the latent heat is increasing
b. the movement of heat is rapid
c. there is no movement of heat
d. the latent heat is decreasing
e. the movement of heat is slow
Answers
There is no movement of heat. The Zeroth Law of Thermodynamics states that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
What is Thermodynamics?Thermodynamics is the study of energy and its transformations. It examines the relationships between heat, work, and other forms of energy. The fundamental laws of thermodynamics provide a framework for studying the behavior of systems, whether they are physical, chemical, or biological in nature. Thermodynamics helps us understand the behavior of gases, liquids, and solids and how they interact with each other. It also provides us with a way to analyze how energy is transferred and used in various processes.
In other words, no heat transfer takes place between two systems that are in thermal equilibrium.
To learn more about Thermodynamics
https://brainly.com/question/27386894
#SPJ4
When bonding, atoms seek to achieve a stable-
A.
noble gas configuration.
B.
halogen gas configuration.
C.
low ionization energy configuration.
D.
high electronegativity configuration
Answers
When bonding, atoms try to achieve a stable noble gas configuration.
Atoms are always trying to achieve stability. In the modern periodic table, noble gases present in the group 18 are the most stable. They have a completely filled outermost or the valence shell, that is, it contains 8 valence electrons in its valence shell.
Due to this stable electronic configuration, they are inert in nature, that is, they do not have a tendency to react by sharing or losing their electrons. When participating in a bonding, atoms try to lose or gain electrons to reach their nearest noble gas configuration and attain stability.
To know more about noble gas here
https://brainly.com/question/11414033
#SPJ4
A controlled experiment _____.Group of answer choicesIs repeated many times to ensure that the results are accurateProceeds at a slow pace to guarantee that the scientist can carefully observe all reactions and process all experimental dataIncludes at least two groups, one of which does not receive the experimental treatmentIncludes at least two groups, one differing from the other by two or more variables
Answers
An experimental group and a control group are both included in a controlled experiment. At least one of the two groups—the group that is not subjected to the experimental treatment—is present in a controlled experiment.
In controlled experiment, the researcher wants to determine how one or more variables affects on something. Since many different variables affecting the results, and most of them are not of interest to the researcher, and they will kept under control. They must remain constant. There must be at least of two groups. One of them is control group, and the other one is experimental group.
During experiment, a researcher compares between two groups. Both are composed of individuals which is coming from same population, so they are very identical in all the aspects except for independent variables that the researcher changes in to the experimental group and they observe that how they will affect the individuals. Control group is used to identify any of the other factors influencing the results which is obtained in the study, apart from the modified variables of the treatment. Independent variable keeps constant in control group.
The experimental group receives the treatment. The researcher voluntarily modifies the independent variable's values to see how it impacts the subjects. There can be several experimental groups.
To know more about experimental here
https://brainly.com/question/28540297
#SPJ4
PLS ANSWER ASAP The following reaction occurs in a car’s catalytic converter.
2NO(g) + 2CO(g) ----> N2(g) + 2CO2(g)
Which answer BEST describes the reducing and oxidizing processes in this reaction?
a. NO and CO are both reducing agents.
b. NO and CO are both oxidizing agents.
c. The oxidation state of nitrogen in NO changes from +2 to 0, and the oxidation state of carbon in CO changes from +2 to +4 as the reaction proceeds.
d. The oxidation state of nitrogen in NO changes from 0 to +2, and the oxidation state of carbon in CO changes from +4 to +2 as the reaction proceeds.
Answers
Answer:
Its B
Explanation: I did the test passed btw
two forces are applied to a large box.one force is pushing it to the right and another equal force is also pushing it the right. which is true about the box's movement?
Answers
Answer: Not moving at all?
Explanation:
...
If 100.0 grams of C3H8 burns, how many moles of carbon dioxide will form?
Answers
Answer:
6.82 Mol CO2
Explanation:
Which statement is most likely correct about the average temperatures in San Francisco, California as compared to Norfolk, Virginia?
It is higher in Norfolk because of the cool ocean currents from the north
It is higher in Norfolk because of the warm ocean currents from the south
It is lower in San Francisco because of the cool ocean currents from the south
It is lower in San Francisco because of the warm ocean currents from the north
Answers
Answer:
It is higher in Norfolk because of the warm ocean currents from the south
Explanation:
Can I have brainliest answer please?
Calculate the volume that 0.540 mol of propane (C3H8) occupies at STP.
Answers
Answer:
i got 12.1 as my answer hope it is right
The the volume that 0.540 mol of propane (C3H8) occupies at STP is 12.1 l.
What is volume ?Volume is a measurement of three-dimensional space that is occupied. It is frequently expressed quantitatively using SI-derived units, as well as several imperial or US-standard units. Volume and the notion of length are connected.
The molar volume (Vm) is the volume occupied by one mole of a chemical element or chemical compound at standard temperature and pressure (STP). You may figure it out by dividing the mass density () by the molar mass (M).
From the ideal gas law; V = nRT/ P
where,
n = 0.540 mol
universal gas constant R = 8.314 J/mol·K
standard temperature T = 273.15 K
standard pressure P = 101 325 Pa
Substituting the values into the formula ;
= 0.540 × 8.314 × 273.15 / 101.325
= 0.0121 m³
= 12.1 l
Thus, The the volume that 0.540 mol of propane (C3H8) occupies at STP is 12.1 l.
To learn more about the volume, follow the link;
https://brainly.com/question/13338592
#SPJ2
salt brine in known to deteriorate steel piping system. what substance can be used to counteract ther corrosuve effects
Answers
Steel piping systems have a history of degrading under salt brine. It is possible to neutralize their corrosive effects by using sodium dichromate.
For melting snow and ice on sidewalks, parking lots, and roads, salt brine is a practical liquid solution. When the salt content of the water reaches a certain proportion, salt brine is produced in tanks that circulate certain volumes of water and rock salt. After that, the solution is pumped into holding tanks in order to be used. In order to address particular temperature ranges of a storm and improve the application's efficacy, other components like calcium or magnesium may be added.
In advance of winter weather, surfaces are pre-treated with salt brine. If Salt Brine is used ahead of a winter storm, it will start to function as soon as the first snowflake falls and assist prevent the accumulation of snow and ice on the pavement.
Learn more about salt brine here:
https://brainly.com/question/28176201
#SPJ4
regardless of where you live and what tempurature it is outside, you should always start the vehicle and give it a few minutes to warm up
Answers
The statement given is true. No matter where you live or what the outside temperature is, you should always start your vehicle and let it warm up for a few minutes.
Define temperature.An object's thermal energy content is measured by its temperature, while heat is the movement of thermal energy between objects of different temperatures.
The average kinetic energy of all the atoms or molecules that make up matter defines temperature in chemistry. Not all materials have the same kinetic energy. A particle can be described using the kinetic energy distribution of the particle at a point in time.
To know more about temperature visit:
https://brainly.com/question/28458734
#SPJ4
The complete question is as follows:
Regardless of where you live and what temperature it is outside, you should always start the vehicle and give it a few minutes to warm up. (True/False)
The normal boiling point for acetone is 56.5°C. At an elevation of 5300 ft the atmospheric pressure is 630. torr. What would be the boiling point of acetone (ΔHvap 32.0 kJ/mol) at this elevation? What would be the vapor pressure of acetone at 22.0°C at this elevation?
Answers
The normal boiling point for acetone is 56.5°C. At an elevation of 5300 ft the atmospheric pressure is 630. torr. The vapor pressure of acetone at 22.0°C at this elevation is 212.22 torr.
What is vapor pressure ?The equilibrium pressure of a vapour above its liquid (or solid), or the pressure of the vapour produced by the evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container, is the vapour pressure of a liquid. Substance; vapour pressure at 25 °C, as examples.
In Step 1:
The normal boiling point for acetone = 56.5°C.
At an elevation of 5300 ft the atmospheric pressure = 630 torr
ΔHvap = 32.0 kJ/mol
The temperature is 24.0 °C
In Step 2:
ln(p1/p2) = (∆ H/R) (1/T2 - 1/T1)
Normal atmospheric pressure
= 1 atm
= 760 torr
So, (760/630) = ((32.000 J/mol) / (8.314 J/K*mol)) (1/T2 - 1/329.65 K).
T2 = 324.4 K = 51.25 °C.
This is the boiling point of acetone at 5300 ft.
The vapor pressure = atmospheric pressure at the boiling point for any liquid.
ln (Pv/630atm) = ((32.000 J/mol) / (8.314 J/K*mol)) (1/324.4 K - 1/297.15)
ln (Pv/630) = -13615388.3
Pv = 212.22 torr
Thus, The vapor pressure of acetone at 22.0°C at this elevation is 212.22 torr.
To learn more about the vapor pressure, follow the link;
https://brainly.com/question/11864750
#SPJ1
Draw two isomers of this, and name each one: C4H6
Answers
Two isomers are Butadiene Bicyclobutane.
What is isomers?Isomers are substances that contain precisely the same number of atoms, i.e., they have the exact same empirical formula, but they differ from one another by the arrangement of the atoms. Ethylbenzene, m-xylene, p-xylene, and o-xylene are a few isomers with the formula C8H10.The term "isomer" refers to molecules or polyatomic ions that have different configurations of atoms in space but the same number of atoms in their molecular formulas, or the number of atoms in each element. Isomerism is the presence or potential for isomers. Isomers may or may not have comparable chemical or physical properties.Isomerism results from the ability of atoms in a molecular formula to be organized in different ways, resulting in compounds with diverse physical and chemical properties. They number two.To learn more about isomers refers to:
brainly.com/question/26298707
#SPJ1
A particular brand of gasoline has a density of 0.733 g/mL at 25 oC. How many grams of this gasoline would fill a 10.7 gal tank (1 US gal
Answers
For a certain brand of gasoline, which has a density of 0.733 g/mL at 25 oC, 41848 grammes would fill a 10.7 gal tank. Mass in relation to volume is referred to as density.
Although the Latin letter D can also be used, the symbol for density is most frequently written as (the lower case Greek letter rho). Any combination of gases can be considered a gas gas solution. As a result of their great distance from one another, gas molecules typically only interact very little. All gases can therefore be thought of as being soluble in one another.
15x 3785.41 ml = 56781.15 ml. Consequently, the mass is calculated as 0.737g/ml x 56781.15 ml = 41848 grammes.
Learn more about molecules here
https://brainly.com/question/19556990
#SPJ4
You travel 35 meters in 26 seconds, what is your average speed?
Answers
Answer:
1.346 meters per second
Explanation:
You traveled 35 meters in 26 seconds
35 / 26 = avg speed.
35 / 26 = 1.346...
If I contain 5 moles of gas in a container with a volume of 62 liters and at a temperature of 222 K, what is the pressure inside the container?
Answers
The pressure inside the container with 5 moles of gas, 62 liters volume and 222K temperature is 1.46 atm.
Ideal gas law illustrates the relation between pressure, volume and temperature of a gas. It is represented by the equation
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the gas constant and T is the temperature.
V = 62 liters
n = 5 moles
R = 0.0821 L(atm) mol⁻¹K⁻¹
T = 222K
PV = nRT
P × 62 = 5 × 0.0821 × 222
P = 5 × 0.0821 × 222/62
P = 1.46 atm
To learn more about ideal gas law here
https://brainly.com/question/27870704
#SPJ4
Consider the balanced reaction below:
2N205 → 4NO2 + O2
How many moles of N205 are needed
to produce 7.90 g of NO2?
[?] moles N205
Answers
0.0859 mol N2O5
Explanation:
First convert the # of grams of NO2 to moles:
7.90 g NO2 × (1 mol NO2/46.0055 g NO2)
= 0.172 mol NO2
Use the molar ratio to find the # moles of N2O5
0.172 mol NO2 × (2 mol N2O5/4 mol NO2)
= 0.0859 mol N2O5
Pure water at 25°C has a pH of
(1) 1 (2) 5 • (3) 7
(4) 14
Answers
Answer:
7. .........................
the highest concentration of life extorts in the top 200 meters of ocean water what is the most important factor that influences this concentration of life
Answers
1.____________________ is the amount of energy that it takes to raise the temperature of 1 gram of a substance by 1 degree kelvin
Answers
Answer: 1joule
Explanation:
Please Help :,D
43.5-g of cesium explosively reacts with water to form hydrogen gas and cesium hydroxide. How many moles of hydrogen gas were formed?
2Cs(s) + 2H2O(l) --> 2CsOH(s) + 1H2(g)
Calculate the mass of silver needed to react with chlorine to produce 42 g of silver chloride.
2Ag(s) + Cl2(g) --> 2AgCl(s)
Silver Nitrate reacts with sodium chloride to make the silver chloride and sodium nitrate. When 12.65 grams of silver nitrate is reacted. How many grams of silver chloride are formed?
AgNO3(s) + NaCl --> AgCl(s) + NaNO3(aq)
Calcium Carbonate decomposes into calcium oxide and a common gas, carbon dioxide. When 91.0 grams of calcium oxide is formed how many liters of carbon dioxide gas is also formed from this reaction. First find the number of moles of carbon dioxide gas actually formed in the reaction, then use the following conversion factor: 1 mol CO2(g) = 22.4 Liters of CO2(g)
CaCO3(s) + HEAT --> CaO(s) + CO2(g)
Answers
Answer:
See the attached images for solution.
When copper wire is placed into a silver(I) nitrate solution, silver crystals and copper(I) nitrate solution form. If a 20.0g sample of copper is used, determine the theoretical yield of silver. If 60.0g silver is actually recovered, determine the percent yield of the reactions.
Answers
Answer:
Theoretical yield of silver = 67.97 g Ag
Percent yield of the reaction = 88.3%
Explanation:
This reaction forms copper(II) nitrate instead of copper(I) nitrate.
First, we have to write the balanced chemical equation for the reaction of copper (Cu) with silver(I) nitrate (AgNO₃) to give silver (Ag) and copper(I) nitrate (CuNO₃):
Cu(s) + 2AgNO₃(aq) → 2Ag(s) + Cu(NO₃)₂(aq)
From the equation, 1 mol of Cu(s) produces 2 moles of Ag(s). We convert the moles to mass with the molecular weight (MW) of the compounds:
MW(Cu) = 63.5 g/mol
mass of Cu = 1 mol Cu x 63.5 g/mol = 63.5 g Cu
MW(Ag) = 107.9 g/mol
mass of Ag = 2 mol Ag x 107.9 g/mol = 215.8 g Ag
Thus, we have the conversion factor: 215.8 g Ag/63.5 g Cu
- From 20.0 g Cu, the following amount of Ag will be obtained:
20.0 g Cu x 215.8 g Ag/63.5 g Cu = 67.97 g Ag (theoretical amount)
- For an actual amount of 60.0 g of Ag, we calculate the percent yield as follows:
%yield = actual amount/theoretical amount x 100 =
= 60.0 g/67.97 g x 100 = 88.3 %
Calculate the volume of 0.25 mol of hydrogen gas at room temperature and pressure
Answers
Answer:
At STP, 1 mole of gas occupies 22.4 L. That means 0.25mol of H2 gas will take up 5.6 L of space.
1. If there is a switch it needs to be turned on. If not, the circuit will be
A. Open
B. Closed
C. Frozen
D. All of the above
Answers
Answer:
The Circuit will be OPEN since the current source is disconnected.
So if its not turned on... The circuit remains Open
Answer
Option A.
What is the molar it's if the HBr solution if 0.500 liters are titrated to an endpoint by 0.100 liters of a 2.00 M KOH solution
Answers
Answer:
0.4 M
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
HBr + KOH —> KBr + H₂O
From the balanced equation above,
The mole ratio of the acid, HBr (nₐ) = 1
The mole ratio of the base, KOH (n₆) = 1
Finally, we shall determine the molarity of HBr. This can be obtained as follow:
Volume of acid, HBr (Vₐ) = 0.5 L
Volume of base, KOH (V₆) = 0.1 L
Molarity of base, KOH (M₆) = 2 M
Molarity of acid, HBr (Mₐ) =?
MₐVₐ / M₆V₆ = nₐ/n₆
Mₐ × 0.5 / 2 × 0.1 = 1
Mₐ × 0.5 / 0.2 = 1
Cross multiply
Mₐ × 0.5 = 0.2
Divide both side by 0.5
Mₐ = 0.2 / 0.5
Mₐ = 0.4 M
Thus, the molarity of the HBr solution is 0.4 M
the data in the table show the amount of 500 g sample of sodium-24 over time. make a graph of the data (remember to label all axes and title the graph)
Answers
The amount of sodium-24 decreases to approximately 125 g.
What is sodium?Sodium is a chemical element with the symbol Na on the periodic table. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metal group of chemical elements.
The graph below shows the amount of 500 g sample of sodium-24 over time. The x-axis of the graph represents time (in hours), and the y-axis represents the amount of sodium-24 (in grams). The title of the graph is "Amount of sodium-24 in 500 g Sample Over Time".
The graph shows that the amount of sodium-24 in the sample decreases over time. After 24 hours, the amount of sodium-24 in the sample decreases to approximately 375 g. After 48 hours, the amount of sodium-24 decreases to approximately 250 g. After 72 hours, the amount of sodium-24 decreases to approximately 125 g.
To learn more about sodium
https://brainly.com/question/25597694
#SPJ1
Use the drawing to help explain why gas pressure decreases when gas is removed from acontainer with a fixed volume
Answers
When gas is removed from a container with a fixed volume, the number of gas particles decreases, so there are fewer collisions and thus less pressure.
Additionally, the remaining gas particles have more space to move around, which also leads to a decrease in pressure. This can be visualized in the drawing by the decrease in the number of gas particles and the increased distance between them as gas is removed from the container.
As the gas particles decrease, there are fewer particles to collide with the walls of the container and therefore less force is exerted on the walls. This results in a decrease in the pressure inside the container.
Read more about the behavior of gases:
https://brainly.com/question/13649307
#SPJ4
A scientist is studying the liquid shown here. She thinks the liquid is a mixture.
Describe an investigation she could do to demonstrate that the liquid is in fact a
combination of substances.
100
1300
200
100
800-m
Answers
Answer:
You have not shown the liquid which is to be shown, please show us
Determine the hydronium ion concentration
in a solution that is 0.00011 M Ca(OH)2
Answers
Answer:
4.5x10⁻¹¹M is the hydronium ion concentration
Explanation:
To solve this concentration we must find, as first, the molar concentration of OH-, [OH-]. With [OH-] we can find pOH, pH, and [H+], hydronium ion concentration, as follows:
[OH-]:
1mol of Ca(OH)2 contains 2 moles of OH-:
0.00011M Ca(OH)2 * (2moles OH- / 1mol Ca(OH)2) = 0.00022M OH-
pOH:
pOH = -log[OH-]
pOH = 3.658
pH:
pH = 14-pOH
pH = 10.34
[H+]:
10^-pH
4.5x10⁻¹¹M is the hydronium ion concentration